Broad Research Interests:
Cancer Biology, Cell Migration, Breast Cancer, Metastasis, Angiogenesis, Cell signaling
Research Summary:
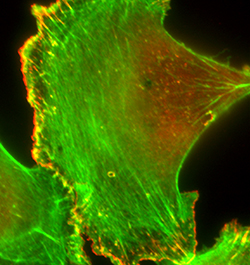
Directed cell migration plays an important role in embryonic development, wound healing, angiogenesis, immune response, cancer invasion and metastasis. Dynamic reorganization of actin cytoskeleton, a key aspect of cell migration, is regulated by the concerted actions of various classes of actin-binding proteins (ABPs), and some of these ABPs are fundamental drivers of actin-based cell motility. Altered expressions and activities of fundamental drivers of cell migration lead to aberrant cell motility in pathologic scenarios. Our main research interests are to: a) gain novel insights on how dysregulation of fundamental drivers of cell migration contributes to metastatic progression of solid cancers and pathological angiogenesis; and b) develop translational strategies exploiting the pathways of dysregulation as a means to suppress metastatic phenotype of cancer cells and angiogenesis-dependent pathology. A major aspect of our research is focused on studying how alteration in profilin (a G-actin binding protein that is important for actin cytoskeletal regulation and many actin-dependent cellular processes) expression and/or function impacts breast cancer metastasis and angiogenesis. In this overall context, we are also exploring novel post-translational modifications of ABPs and how these modifications impact protein function and actin-dependent biological processes.
Another essential feature for remodeling of the actin cytoskeleton during cell motility is the regulation of de novo synthesis of ABPs and other actin cytoskeleton-associated proteins in response to motility cues. Serum-response factor (SRF), a ubiquitously expressed and highly conserved transcription factor, is a critical player in this process. SRF binds to the CArG [CC(AT)6GG)] consensus sequence located on a variety of genes including SRF itself and many of those involved with the actin cytoskeleton, cell-matrix/cell-cell adhesion, and cellular contractility. Myocardin-related transcriptional coactivator MRTF (also known as MKL) plays a key role in linking actin dynamics to SRF-mediated transcriptional regulation of genes. We are also actively pursuing the biology of MRTF family transcriptional coactivators in physiological and pathological contexts.
For these studies, we use a variety of experimental approaches including mammary gland-related techniques, RNAi, 2D gel electrophoresis, genetically engineered mouse models of cancer, tumor xenografts, in vitro and in vivo angiogenesis assays, functional genomics and proteomics, live cell imaging and small molecule screening.