Overview
The Soft Materials and Rheology Group works with polymers, interfaces, smart materials, buckling phenomena, emulsions, colloids, and the rheology of complex materials. Here are some ongoing projects
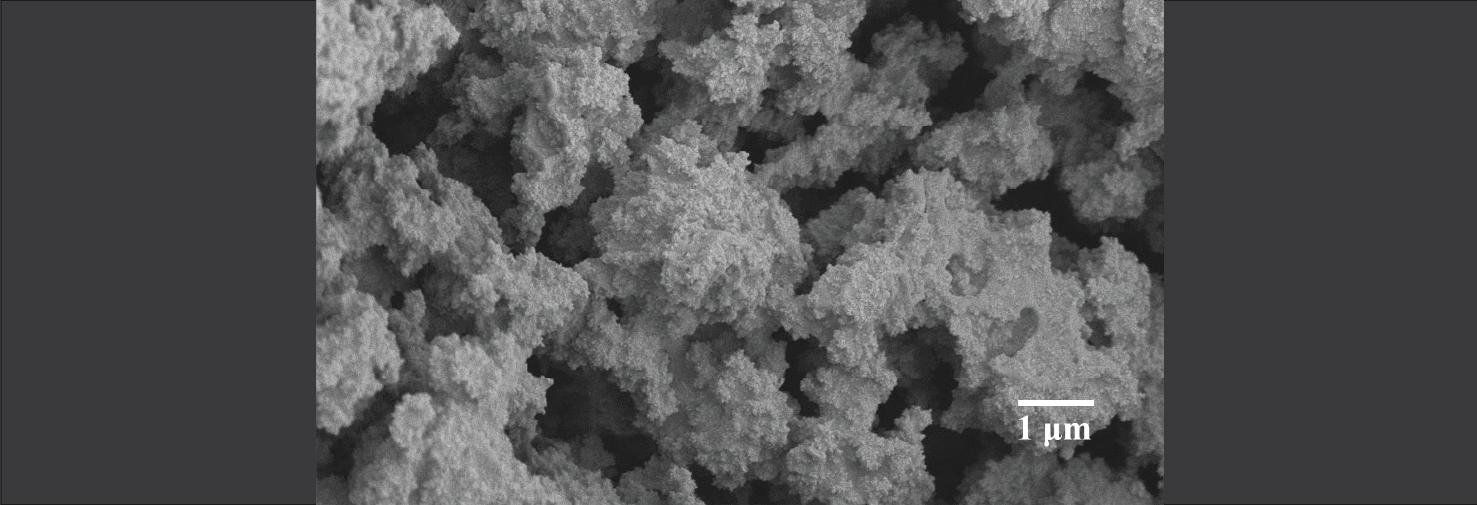
Capillary phenomena in particulate systems
Dry sand can be poured out of a bucket, whereas wet sand can be molded into sandcastle. This is the most familiar example of capillary phenomena inducing a change in structure and flow behavior of particulate systems. The Velankar group has a long-standing interest this area, examining particles at interfaces in emulsions or in polymer blends, using capillary forces to aggregate particles in a suspension, and examining the morphology and flow behavior of particle-filled blends of immiscible polymers.
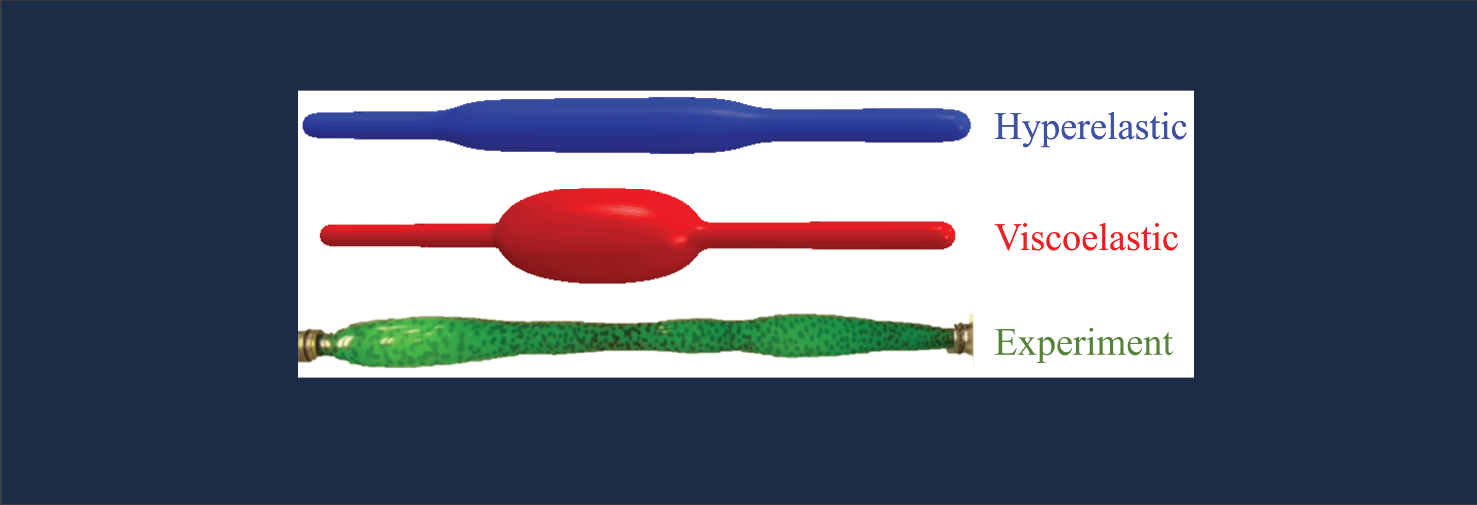
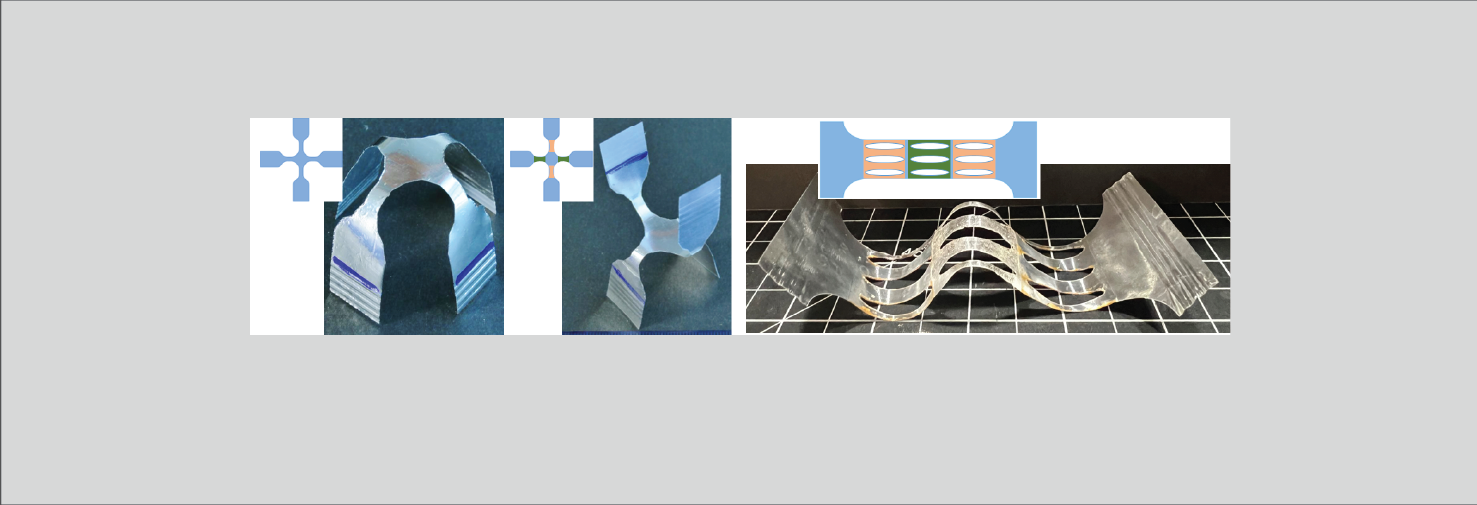
Mechanics of soft materials
Wrinkled skin is an excellent example of how a stiff film bonded to an soft foundation responds to compression. We have examined numerous example of such buckling instabilities including buckling of films on viscous liquids, swelling-induced folding of soft elastomeric films, surface creasing of a viscoelastic layer, and plasticity effects in wrinkle formation. Other problems of interest include necking and cracking of elastic-plastic layers and mechanics of plastic tubes. Also related to this is our ongoing research on the wrinkled morphology of arteries, and developing anti-fouling surfaces based on topographic morphing.
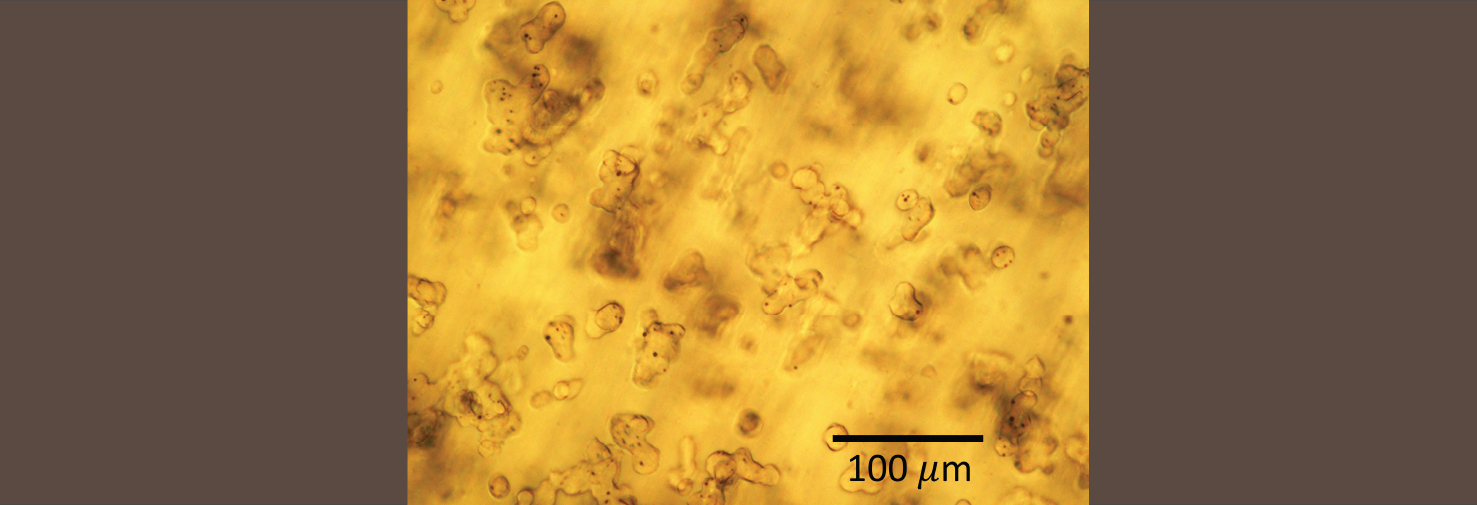
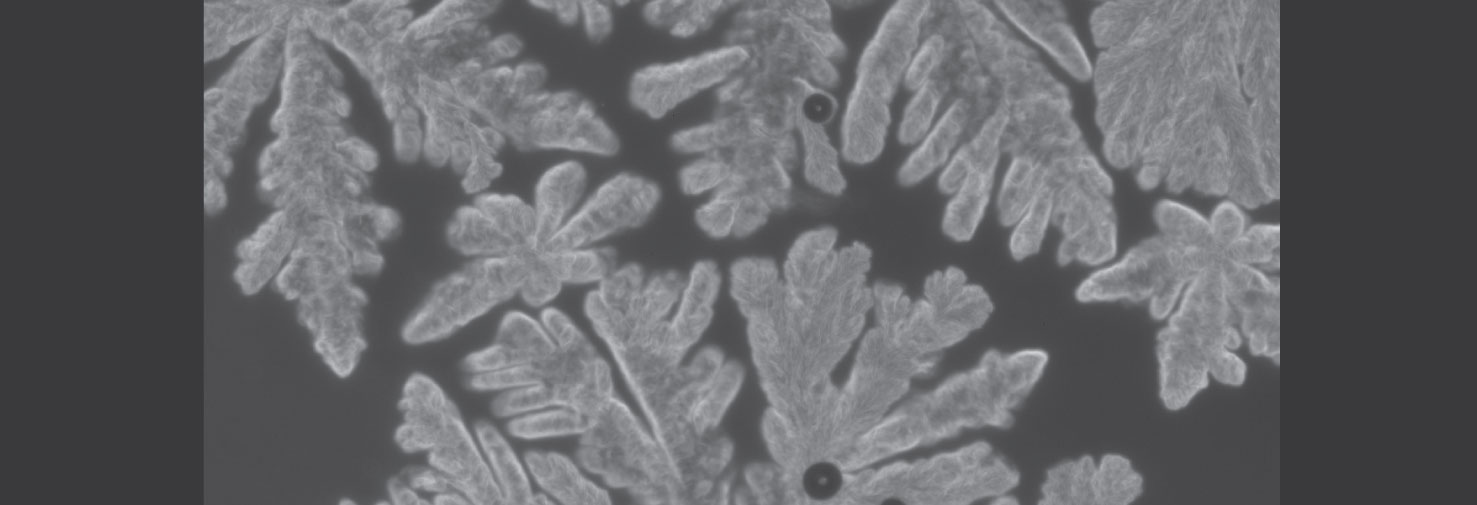
Crystallization of a polymer hydrate
The polymer polyoxacyclobutane can cocrystallize with water to form a polymer hydrate. This is a rare example of a polymer co-crystallizing with a small molecule, and even more remarkable because the polymer is actually hydrophobic. We are examining the thermodynamics and crystallization kinetics of such hydrates