Surface coating for neural implants
Surface coating for neural implants
Implantable neural implants offer high spatial and temporal resolution useful for recording long-term neural signals. Current arrays encounter significant reliability and stability issues over long-term implantation, and a major contributor is the undesired host tissue responses. Several approaches are being explored in our lab to modify the device surface for improved neural electrode-tissue interface, by promoting neuronal health and adhesion while suppressing glial activation and scar formation. The two categories of coatings are:
- Bioactive coatings
- Our most mature bioactive coating is the neuronal adhesion molecule L1 coating which can be covalently immobilized onto silicon and parylene C based surfaces and promote neurite attachment while reducing glial encapsulation. Importantly, L1 coating greatly improves single unit recording yield in vivo. Adding a thiolated nanoparticle base layer helps to stabilize and maintain the bioactivity of the coating, enabling dissemination to user labs. The L1 coating has been extensively tested in rodent models and is currently being investigated in non-human primate models in our collaborator’s labs.
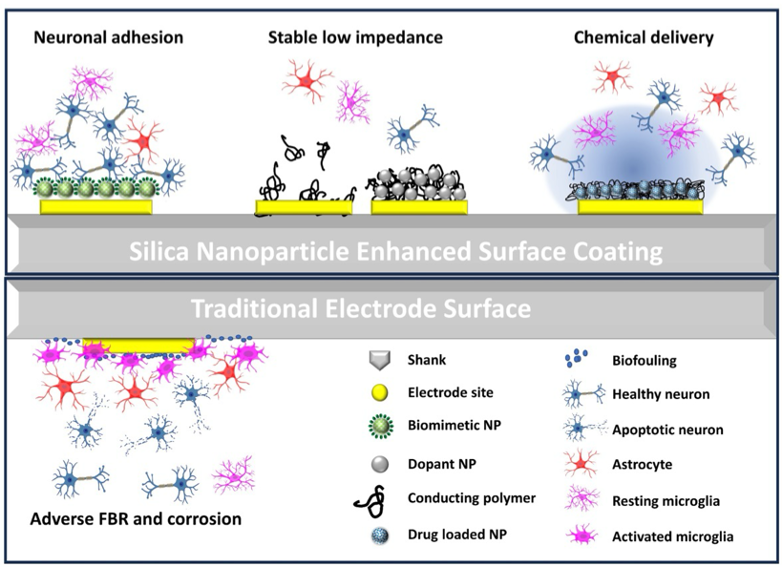
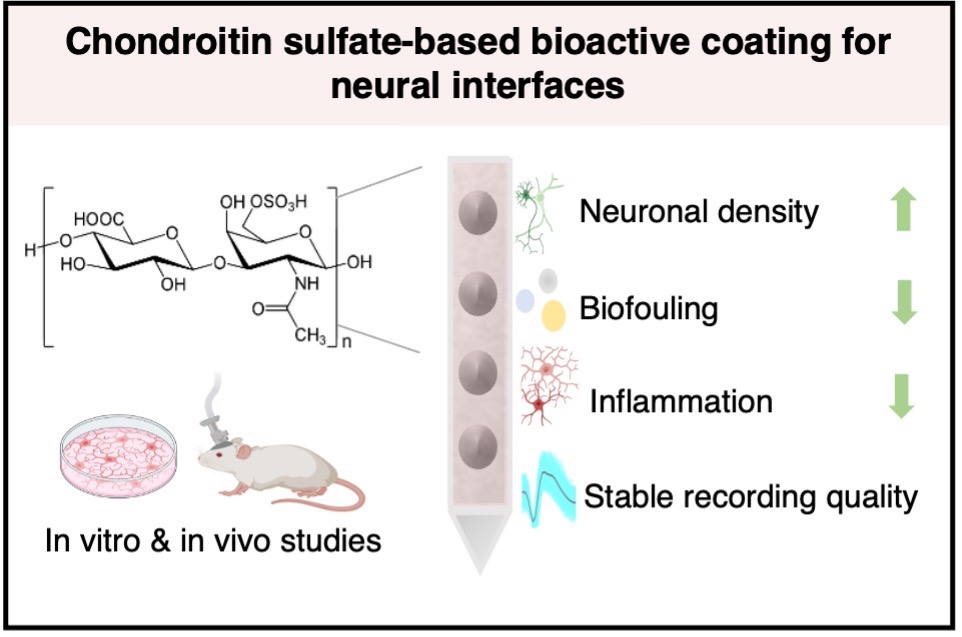
- Chondroitin-sulfate is a hydrophilic carbohydrate that provides an anti-fouling and neurite-promoting surface, which leads to milder inflammatory response in vitro and in vivo and demonstrates improved recording performance for acute implantation in vivo.
- Synthetic polymer coatings
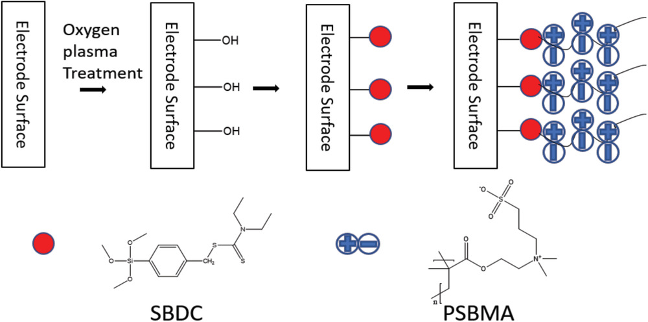
- Synthetic zwitterionic coating, comprised of catechol-poly(sulfobetaine methacrylate) (PSBMA) with polydopamine (PDA) can be covalently grafted onto neural probe surface via photoiniferter-mediated polymerization. Once deposited, the coated surface offers a stable, anti-fouling surface which can reduce microglia end-feet spreading in vivo. We are currently investigating how these benefits translate to the recording performance of coated MEAs.
Relevant publications:
- D Shi, S Narayanan, K Woeppel, XT Cui. Improving the Biocompatibility and Functionality of Neural Interface Devices with Silica Nanoparticles. Acc. Chem. Res. 2024, 57, 12, 1684–1695. doi:10.1021/acs.accounts.4c00160
- Woeppel K, Dhawan V, Shi D, Cui XT. Nanotopography-enhanced biomimetic coating maintains bioactivity after weeks of dry storage and improves chronic neural recording. Biomaterials. 2023;302:122326. doi:10.1016/j.biomaterials.2023.122326
- Kushwah N, Woeppel K, Dhawan V, Shi D, Cui XT. Effects of neuronal cell adhesion molecule L1 and nanoparticle surface modification on microglia. Acta Biomater. 2022;149:273-286. doi:10.1016/j.actbio.2022.06.038
- Ding R, Miller NC, Woeppel KM, Cui XT, Jacobs TDB. Surface Area and Local Curvature: Why Roughness Improves the Bioactivity of Neural Implants. Langmuir. 2022;38(24):7512-7521. doi:10.1021/acs.langmuir.2c00473
- Woeppel KM, Cui XT. Nanoparticle and Biomolecule Surface Modification Synergistically Increases Neural Electrode Recording Yield and Minimizes Inflammatory Host Response. Adv Healthc Mater. 2021;10:2002150. doi:10.1002/adhm.202002150
- Golabchi A, Woeppel KM, Li X, Lagenaur CF, Cui XT. Neuroadhesive protein coating improves the chronic performance of neuroelectronics in mouse brain. Biosens Bioelectron. 2020;155:112096. doi:10.1016/j.bios.2020.112096
- Cody PA, Eles JR, Lagenaur CF, Kozai TDY, Cui XT. Unique electrophysiological and impedance signatures between encapsulation types: An analysis of biological Utah array failure and benefit of a biomimetic coating in a rat model. Biomaterials. 2018;161:117-128. doi:10.1016/j.biomaterials.2018.01.025
- Woeppel KM, Zheng XS, Cui XT. Enhancing surface immobilization of bioactive molecules via a silica nanoparticle based coating. J Mater Chem B. 2018;(19):[page range]. doi:10.1039/C8TB00408K
- Eles JR, Vazquez AL, Snyder NR, Lagenaur C, Murphy MC, Kozai TDY, Cui XT. Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy. Biomaterials. 2017;113:279-292
- Kolarcik CL, Bourbeau D, Azemi E, Rost E, Zhang L, Lagenaur CF, Weber DJ, Cui XT. In vivo effects of L1 coating on inflammation and neuronal health at the electrode–tissue interface in rat spinal cord and dorsal root ganglion. Acta Biomater. 2012;8(10):3561-3575. doi:10.1016/j.actbio.2012.06.034
- Azemi E, Lagenaur CF, Cui XT. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 2011;32(3):681-692. doi:10.1016/j.biomaterials.2010.09.033
- Azemi E, Stauffer WR, Gostock MS, Lagenaur CF, Cui XT. Surface immobilization of neural adhesion molecule L1 for improving the biocompatibility of chronic neural probes: In vitro characterization. Acta Biomater. 2008;4(5):1208-1217. doi:10.1016/j.actbio.2008.05.002
- Dhawan V, Martin PN, Hu X, Cui XT. Investigation of a chondroitin sulfate based bioactive coating for neural interface applications. J. Mater. Chem. B, 2024. DOI: 10.1039/d4tb00501e.
- Wu B, Castagnola E, Cui XT. Zwitterionic Polymer Coated and Aptamer Functionalized Flexible Micro-Electrode Arrays for In Vivo Cocaine Sensing and Electrophysiology. Micromachines. 2023; 14(2):323. doi: 10.3390/mi14020323
- Yang Q, Wu B, Eles JR, Vazquez AL, Kozai TDY, Cui XT. Zwitterionic Polymer Coating Suppresses Microglial Encapsulation to Neural Implants In Vitro and In Vivo. Adv Biosyst. 2020;4:1900287. doi:10.1002/adbi.201900287
- Golabchi A, Wu B, Cao B, Bettinger CJ, Cui XT. Zwitterionic polymer/polydopamine coating reduce acute inflammatory tissue responses to neural implants. Biomaterials. 2019;225:119519. doi:10.1016/j.biomaterials.2019.119519
